Why IRB Approval Is Important and How to Take It|2025
Learn why IRB approval is important and how to take it. Discover the process of obtaining Institutional Review Board approval to ensure ethical standards in your research involving human participants.
Research ethics are an essential component of any study involving human participants, and the process of securing Institutional Review Board (IRB) approval is a fundamental part of this. IRB approval ensures that research is conducted responsibly, ethically, and safely, protecting participants from harm and ensuring that research findings are credible and trustworthy. This paper will delve into the importance of IRB approval, how to obtain it, the types of research that require IRB approval, and various aspects related to the approval process.
What is an Institutional Review Board (IRB)?
An Institutional Review Board (IRB) is a group of individuals tasked with reviewing and overseeing research involving human participants to ensure that it meets ethical standards. IRBs are essential in safeguarding the rights and welfare of research participants, especially when research involves potentially sensitive topics or vulnerable populations.
The IRB’s primary function is to review research proposals and ensure that the study will be conducted in a manner that protects the rights, dignity, and safety of the participants. This review process involves a careful assessment of the study design, data collection methods, informed consent procedures, and risk management plans. The IRB also evaluates whether the research complies with ethical guidelines such as those set forth by the U.S. Department of Health & Human Services (HHS) and the Food and Drug Administration (FDA).
Why IRB Approval Is Important
There are several key reasons why IRB approval is important in the research process:
- Protects Human Participants: The most crucial role of the IRB is to ensure that participants are treated with respect and that their rights are protected. This includes ensuring that participants are not exposed to undue risks, that they are fully informed about the nature of the research, and that they consent voluntarily without any coercion.
- Ensures Ethical Standards Are Met: Research involving human participants must adhere to ethical principles, including respect for persons, beneficence (doing good), and justice. The IRB ensures that the research complies with these principles by carefully reviewing the proposed methodology.
- Legal and Regulatory Compliance: IRB approval is often mandated by law or institutional policy. For example, studies funded by the U.S. government or involving federal grants require IRB approval to ensure compliance with federal regulations.
- Enhances Research Quality and Credibility: By ensuring that studies follow ethical and methodological guidelines, IRB approval enhances the credibility of the research. Studies that fail to obtain IRB approval may lack validity, and their results could be questioned or rejected by the scientific community.
- Minimizes Risk to Researchers and Institutions: Failing to secure IRB approval can lead to significant legal and reputational consequences for both researchers and their institutions. Non-compliance with ethical standards can lead to lawsuits, loss of funding, and damage to an institution’s reputation.
- Facilitates Trust in Research: Obtaining IRB approval fosters trust between researchers, participants, and the broader public. When participants are assured that their rights are being safeguarded, they are more likely to consent to participate in research. Trust in the ethical conduct of research is crucial for its success.
How to Get IRB Approval
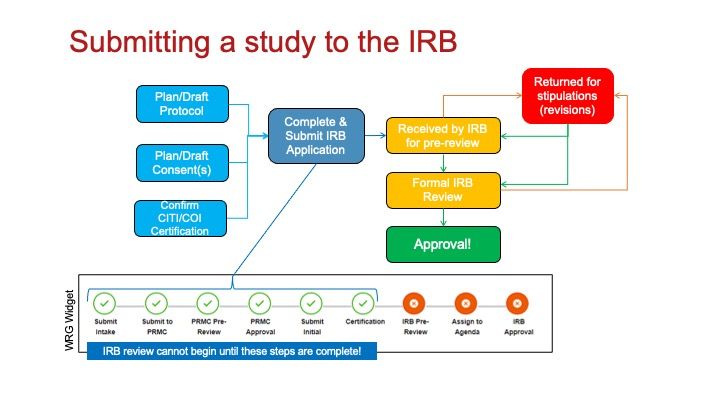
Securing IRB approval is a critical step in the research process, and it requires researchers to follow a specific procedure. The steps involved in obtaining IRB approval typically include the following:
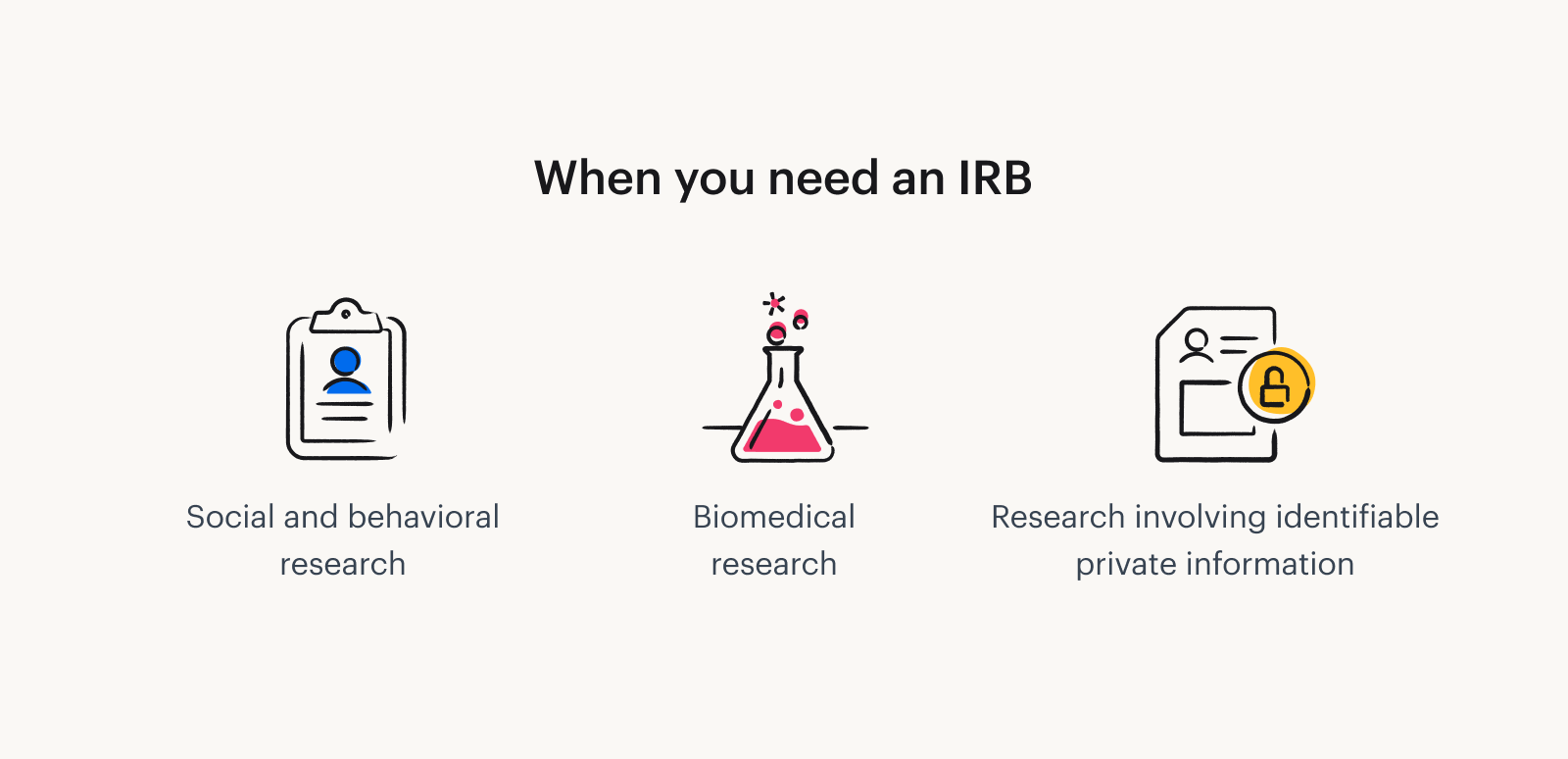
- Determine if IRB Approval is Needed: Before submitting an IRB application, researchers must first determine whether their study requires IRB approval. If a study involves human participants, particularly those involving surveys, interviews, or interventions, it will likely require IRB approval. In some cases, research may be exempt from IRB review, particularly if it involves minimal risk or if the research does not involve identifiable information.
- Prepare Your Research Proposal: Researchers should carefully plan their study and prepare all necessary documentation for submission. This includes a detailed research proposal outlining the study design, objectives, methodology, participant recruitment, data collection methods, and risk mitigation strategies.
- Complete the IRB Application: Most institutions provide an online portal where researchers can submit their IRB applications. This application typically requires researchers to provide details about the research, including the risks involved, the participant consent process, and the methods used to ensure participant confidentiality.
- Informed Consent Process: One of the key components of the IRB application is the informed consent process. Researchers must provide a clear and thorough explanation of the study to participants, including any risks involved, the purpose of the research, and their right to withdraw at any time. Informed consent must be obtained from participants before data collection begins.
- Review and Approval: Once the application is submitted, the IRB will review the proposal to ensure it complies with ethical standards. This review may involve full board review, expedited review, or exemption review, depending on the level of risk involved in the research.
- Address Feedback and Revise: If the IRB identifies any concerns or requires changes to the research design, the researcher must address these issues and resubmit the application. This iterative process ensures that all ethical considerations are taken into account.
- Ongoing Monitoring: After receiving IRB approval, researchers must continue to monitor their study and report any adverse events or deviations from the approved protocol. If significant changes to the study design are needed, a new IRB application may be required.
Do I Need IRB Approval for a Survey?
A common question researchers have is whether they need IRB approval for a survey. The answer depends on several factors, including the nature of the survey and the type of data being collected.
In general, if the survey involves human participants and collects data that can identify individuals or pose potential risks (such as sensitive or personal information), it requires IRB approval. However, if the survey is anonymous and does not collect identifiable information, it may be exempt from review. Researchers should consult their institution’s IRB guidelines to determine whether their survey requires approval.
Which Type of Research Does Not Need to Get IRB Approval?
While most research involving human participants requires IRB approval, there are some exceptions. Research that may not require IRB approval includes:
- Research Involving Only Data, Documents, or Records: Studies that rely solely on existing data, documents, or records that are publicly available or are completely de-identified may be exempt from IRB review.
- Research Involving Publicly Available Data: Research that uses data from publicly available sources, such as government databases, may not require IRB approval as long as no personal or identifiable information is used.
- Certain Types of Educational Research: Research conducted in educational settings that involves normal educational practices, such as the use of instructional strategies or classroom management techniques, may be exempt from IRB review.
Researchers should always check with their institution’s IRB to determine whether their specific research qualifies for exemption.
Do Private Companies Need IRB Approval?
Private companies conducting research that involves human participants must also adhere to ethical standards and seek IRB approval when required. While private companies may not be subject to the same regulatory oversight as academic institutions, they must still follow ethical guidelines when conducting research that involves human participants, especially if the research is funded by federal grants or seeks to publish findings in academic journals.
Private companies, especially those in industries such as healthcare or pharmaceuticals, must obtain IRB approval for clinical trials or other research involving human subjects to ensure compliance with ethical and regulatory standards.
How Long Does It Take to Get IRB Approval?
The time it takes to obtain IRB approval can vary depending on several factors, including the complexity of the study, the level of review required, and the efficiency of the institution’s IRB process. Typically, the approval process can take anywhere from a few weeks to a couple of months. Expedited reviews may take less time, while studies requiring full board review can take longer.
Researchers should allow sufficient time for the approval process when planning their research and consider potential delays in submitting additional documentation or responding to IRB requests.
What Are Some Important Reasons for Having IRBs?
The reasons for having IRBs extend beyond just compliance with laws and regulations. Some key reasons include:
- Ethical Oversight: IRBs provide an ethical review of research, ensuring that human participants are treated with respect, that their privacy is protected, and that their participation does not result in harm.
- Risk Management: IRBs help to identify and mitigate potential risks in research, ensuring that participants are not exposed to undue harm or distress.
- Public Trust: IRB approval enhances public confidence in the research process. When people know that research is subject to ethical oversight, they are more likely to trust the findings and participate in studies.
- Protection of Vulnerable Populations: IRBs help ensure that vulnerable groups, such as children, prisoners, or individuals with disabilities, are not exploited or exposed to unnecessary risks in research.
- Compliance with Legal and Regulatory Standards: Institutional and federal regulations require IRB approval for many types of research, and IRBs help ensure that researchers meet these requirements.
Conclusion
In conclusion, IRB approval is a vital part of the research process that ensures ethical and responsible conduct when conducting studies involving human participants. Obtaining IRB approval helps to protect participants, enhances the quality and credibility of research, and ensures compliance with legal and regulatory standards. Researchers must carefully follow the steps for obtaining IRB approval and consult their institution’s IRB guidelines to determine when approval is necessary. By understanding and adhering to these guidelines, researchers can contribute to the advancement of knowledge while maintaining the highest ethical standards.
Needs help with similar assignment?
We are available 24x7 to deliver the best services and assignment ready within 3-4 hours? Order a custom-written, plagiarism-free paper